
Sucralfate is a cytoprotective agent, an oral gastrointestinal medication primarily indicated for the treatment of active duodenal ulcers. Sucralfate is the hydrous basic aluminium salt of sucrose Octasulfate, it contains the equivalent of not less than 30.0 % and not more than 38.0 % of sucrose Octasulfate (C12H14O35S8).
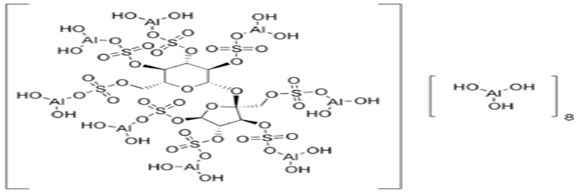
Sucralfate is a medication primarily taken to treat active duodenal ulcers. Sucralfate is also used for the treatment of gastroesophageal reflux disease and stress ulcers. Sucralfate is a sucrose sulfate-aluminium complex that binds to the ulcer, creating a physical barrier that protects the gastrointestinal tract from stomach acid and prevents the degradation of mucus. It also promotes bicarbonate production and acts like an acid buffer with cytoprotective properties.
This medication is used to treat ulcers in the intestines. Sucralfate forms a coating over ulcers, protecting the area from further injury. This helps ulcers heal more quickly. It has almost no adverse side effects besides sometimes causing constipation (which can be used therapeutically in some patients with diarrhea). ... A major disadvantage is that sucralfate can only be given orally, which limits its usefulness in vomiting patients.
Expiry:5 Years after date of manufacture
WHO-GMP: Par Drugs And Chemicals Limited is WHO GMP Certified.
(Issued by Food & Drug Control Administration)
Note: Above Certificate is available upon request.
GMO: The Product does not contain any Genetically Modified Organisms (GMO) of raw materials used in manufacturing of the same are also not genetically modified.
Non-Irradiation:The product was never subjected to any kind of ionized irradiation and contains no radioactivity not even in minor amounts.
Allergens: The product does not contain any of the below mentioned allergens:
Cereals containing Gluten, Crustaceans, Eggs, Fish, Peanuts, Treenuts, Soybeans, Milk, Sesame seeds, Sulphur Dioxide ands sulphites (concentration > 10 mg/kg), Lupin, Molluscs.
TSE/BSE: The Product is not derived from animal/plant origin & hence this product is free from Transmissible Spongiform Encephalopathy (TSE) and Bovine Spongiform Encephalopathy (BSE). It is synthetically derived
Residual Solvent: Class1, Class 2, Class 3 & Table 4 solvents are not used in the production of our product.
Vegetarians / Vegans: Our product is suitable for consumption by vegetarians and vegans.
Store Protected From Moisture
For Stuffing Information click here