
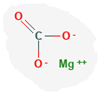
Light Magnesium Carbonate is an inorganic compound with the chemical formula MgCO3. Light Magnesium Carbonate is a common antacid drug that is used Pharmaceutical Aid; Light Magnesium Carbonate contains not less than 40.0 per cent and Not more than 45.0 per cent of MgO.
Magnesite and dolomite minerals are used to produce magnesium metal and basic refractory bricks. Magnesium Carbonate is also used in flooring, fire proofing, fire extinguishing compositions, cosmetics, dusting powder and toothpaste. Other applications are as filler material, smoke suppressant in plastics, a reinforcing agent in neoprene rubber, a drying agent and colour retention in floods. In addition, high purity magnesium carbonate is used as antacid and as an additive in table salt to keep it free flowing.
This medication is a mineral supplement used to prevent and treat low amounts of magnesium in the blood. Magnesium is very important for the normal functioning of cells, nerves, muscles, bones, and the heart. Usually, a well-balanced diet provides normal blood levels of magnesium. However, certain situations cause your body to lose magnesium faster than you can replace it from your diet.
Stomach upset and diarrhea may occur. Taking this product with a meal helps to reduce these effects. If either of these effects persist or worsen, tell your doctor or pharmacistpromptly. If your doctor has directed you to use this medication, remember that he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Expiry:5 Years after date of manufacture
WHO-GMP: Par Drugs And Chemicals Limited is WHO GMP Certified.
(Issued by Food & Drug Control Administration)
Note: Above Certificate is available upon request.
GMO: The Product does not contain any Genetically Modified Organisms (GMO) of raw materials used in manufacturing of the same are also not genetically modified.
Non-Irradiation:The product was never subjected to any kind of ionized irradiation and contains no radioactivity not even in minor amounts.
Allergens: The product does not contain any of the below mentioned allergens:
Cereals containing Gluten, Crustaceans, Eggs, Fish, Peanuts, Treenuts, Soybeans, Milk, Sesame seeds, Sulphur Dioxide ands sulphites (concentration > 10 mg/kg), Lupin, Molluscs.
TSE/BSE: The Product is not derived from animal/plant origin & hence this product is free from Transmissible Spongiform Encephalopathy (TSE) and Bovine Spongiform Encephalopathy (BSE). It is synthetically derived
Residual Solvent: Class1, Class 2, Class 3 & Table 4 solvents are not used in the production of our product.
Vegetarians / Vegans: Our product is suitable for consumption by vegetarians and vegans.
Store Protected From Moisture
20 & 25 kg net weight packed in LDPE Liner follow by HDPE Woven Bag.
For Stuffing Information click here